Posts
Showing posts from 2012
Scientists Produce Hydrogen for Fuel Cells Using an Inexpensive Catalyst Under Real-World Conditions
- Get link
- Other Apps
Self-Charging Power Cell Converts and Stores Energy
- Get link
- Other Apps
Scientists Use Microbes to Make 'Clean' Methane
- Get link
- Other Apps
Clothing the Body Electric: Cotton T-Shirt Fabric Can Store Electricity, Maybe Keep Your Cell Phone Charged
- Get link
- Other Apps
Highly Transparent Solar Cells for Windows That Generate Electricity
- Get link
- Other Apps
ZPlasma: Plasma Startup Creates High-Energy Light to Make Smaller Microchips
- Get link
- Other Apps
The Anatomy Of A Pass, A Quantitative Analysis On Why A VC Passes
- Get link
- Other Apps
Peter Thiel - about cleantech investing
- Get link
- Other Apps
Global Investment in Renewable Energy Powers to Record $257 Billion
- Get link
- Other Apps
Carbon Is Key for Getting Algae to Pump out More Oil
- Get link
- Other Apps
Scientists Generate Electricity from Viruses
- Get link
- Other Apps
Artificial Leaf Device Produces Hydrogen in Water Using Only Sunlight
- Get link
- Other Apps
Graphene Boosts Efficiency of Next-Gen Solar Cells
- Get link
- Other Apps
Solar Cell That Also Shines: Luminescent 'LED-Type' Design Breaks Efficiency Record
- Get link
- Other Apps
Nature's Billion-Year-Old Battery Key to Storing Energy
- Get link
- Other Apps
Artificial Photosynthesis Breakthrough: Fast Molecular Catalyzer
- Get link
- Other Apps
Algae Biofuels: The Wave of the Future
- Get link
- Other Apps
Researchers Produce Environmentally Friendly Surfactants Using Biotechnology
- Get link
- Other Apps
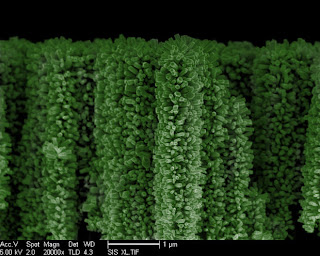
Nanotrees Harvest the Sun's Energy to Turn Water Into Hydrogen Fuel
- Get link
- Other Apps
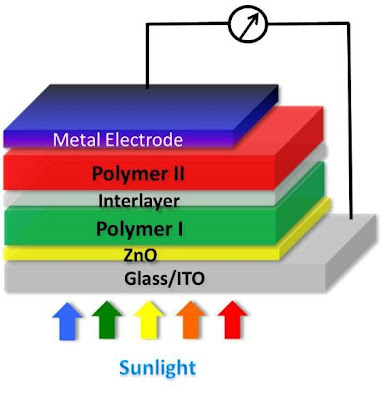
Engineers Create Tandem Polymer Solar Cells That Set Record for Energy-Conversion
- Get link
- Other Apps