Posts
Showing posts from February, 2012
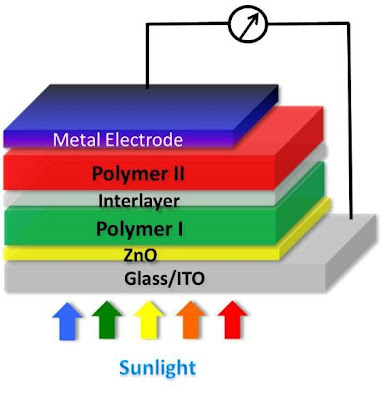
Engineers Create Tandem Polymer Solar Cells That Set Record for Energy-Conversion
- Get link
- Other Apps
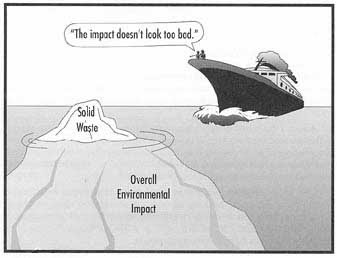
Environmental Impacts Cost 41 Cents for Every $1 of Revenue
- Get link
- Other Apps
New Battery Could Lead to Cheaper, More Efficient Solar Energy
- Get link
- Other Apps
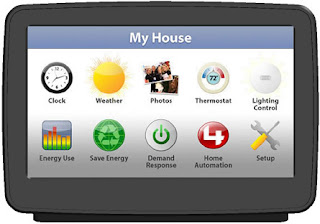
Consumers want better language, design, & layout for energy info
- Get link
- Other Apps
Biosolar Breakthrough Promises Cheap, Easy Green Electricity
- Get link
- Other Apps